Social Neuroscience
By Tiffany A. Ito and Jennifer T. KubotaUniversity of Colorado Boulder, University of Delaware
This module provides an overview of the new field of social neuroscience, which combines the use of neuroscience methods and theories to understand how other people influence our thoughts, feelings, and behavior. The module reviews research measuring neural and hormonal responses to understand how we make judgments about other people and react to stress. Through these examples, it illustrates how social neuroscience addresses three different questions: (1) how our understanding of social behavior can be expanded when we consider neural and physiological responses, (2) what the actual biological systems are that implement social behavior (e.g., what specific brain areas are associated with specific social tasks), and (3) how biological systems are impacted by social processes.
Learning Objectives
- Define social neuroscience and describe its three major goals.
- Describe how measures of brain activity such as EEG and fMRI are used to make inferences about social processes.
- Discuss how social categorization occurs.
- Describe how simulation may be used to make inferences about others.
- Discuss the ways in which other people can cause stress and also protect us against stress.
Psychology has a long tradition of using our brains and body to better understand how we think and act. For example, in 1939 Heinrich Kluver and Paul Bucy removed (i.e. lesioned) the temporal lobes in some rhesus monkeys and observed the effect on behavior. Included in these lesions was a subcortical area of the brain called the amygdala. After surgery, the monkeys experienced profound behavioral changes, including loss of fear. These results provided initial evidence that the amygdala plays a role in emotional responses, a finding that has since been confirmed by subsequent studies (Phelps & LeDoux, 2005; Whalen & Phelps, 2009).
What Is Social Neuroscience?
Social neuroscience similarly uses the brain and body to understand how we think and act, with a focus on how we think about and act toward other people. More specifically, we can think of social neuroscience as an interdisciplinary field that uses a range of neuroscience measures to understand how other people influence our thoughts, feelings, and behavior. As such, social neuroscience studies the same topics as social psychology, but does so from a multilevel perspective that includes the study of the brain and body. Figure 1 shows the scope of social neuroscience with respect to the older fields of social psychology and neuroscience. Although the field is relatively new – the term first appeared in 1992 (Cacioppo & Berntson, 1992) – it has grown rapidly, thanks to technological advances making measures of the brain and body cheaper and more powerful than ever before, and to the recognition that neural and physiological information are critical to understanding how we interact with other people.
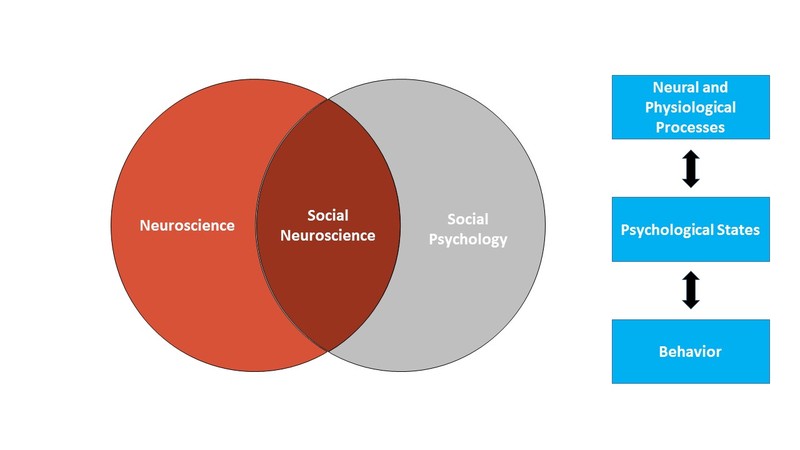
Social neuroscience can be thought of as both a methodological approach (using measures of the brain and body to study social processes) and a theoretical orientation (seeing the benefits of integrating neuroscience into the study of social psychology). The overall approach in social neuroscience is to understand the psychological processes that underlie our social behavior. Because those psychological processes are intrapsychic phenomena that cannot be directly observed, social neuroscientists rely on a combination of measureable or observable neural and physiological responses as well as actual overt behavior to make inferences about psychological states (see Figure 1). Using this approach, social neuroscientists have been able to pursue three different types of questions: (1) What more can we learn about social behavior when we consider neural and physiological responses? (2) What are the actual biological systems that implement social behavior (e.g., what specific brain areas are associated with specific social tasks)? and (3) How are biological systems impacted by social processes?
In this module, we review three research questions that have been addressed with social neuroscience that illustrate the different goals of the field. These examples also expose you to some of the frequently used measures.
How Automatically Do We Judge Other People?
Social categorization is the act of mentally classifying someone as belonging in a group. Why do we do this? It is an effective mental shortcut. Rather than effortfully thinking about every detail of every person we encounter, social categorization allows us to rely on information we already know about the person’s group. For example, by classifying your restaurant server as a man, you can quickly activate all the information you have stored about men and use it to guide your behavior. But this shortcut comes with potentially high costs. The stored group beliefs might not be very accurate, and even when they do accurately describe some group members, they are unlikely to be true for every member you encounter. In addition, many beliefs we associate with groups – called stereotypes – are negative. This means that relying on social categorization can often lead people to make negative assumptions about others.
The potential costs of social categorization make it important to understand how social categorization occurs. Is it rare or does it occur often? Is it something we can easily stop, or is it hard to override? One difficulty answering these questions is that people are not always consciously aware of what they are doing. In this case, we might not always realize when we are categorizing someone. Another concern is that even when people are aware of their behavior, they can be reluctant to accurately report it to an experimenter. In the case of social categorization, subjects might worry they will look bad if they accurately report classifying someone into a group associated with negative stereotypes. For instance, many racial groups are associated with some negative stereotypes, and subjects may worry that admitting to classifying someone into one of those groups means they believe and use those negative stereotypes.

Social neuroscience has been useful for studying how social categorization occurs without having to rely on self-report measures, instead measuring brain activity differences that occur when people encounter members of different social groups. Much of this work has been recorded using the electroencephalogram, or EEG. EEG is a measure of electrical activity generated by the brain’s neurons. Comparing this electrical activity at a given point in time against what a person is thinking and doing at that same time allows us to make inferences about brain activity associated with specific psychological states. One particularly nice feature of EEG is that it provides very precise timing information about when brain activity occurs. EEG is measured non-invasively with small electrodes that rest on the surface of the scalp. This is often done with a stretchy elastic cap, like the one shown in Figure 2, into which the small electrodes are sewn. Researchers simply pull the cap onto the subject’s head to get the electrodes into place; wearing it is similar to wearing a swim cap. The subject can then be asked to think about different topics or engage in different tasks as brain activity is measured.
To study social categorization, subjects have been shown pictures of people who belong to different social groups. Brain activity recorded from many individual trials (e.g., looking at lots of different Black individuals) is then averaged together to get an overall idea of how the brain responds when viewing individuals who belong to a particular social group. These studies suggest that social categorization is an automatic process – something that happens with little conscious awareness or control – especially for dimensions like gender, race, and age (Ito & Urland, 2003; Mouchetant-Rostaing & Giard, 2003). The studies specifically show that brain activity differs when subjects view members of different social groups (e.g., men versus women, Black people versus White peop), suggesting that the group differences are being encoded and processed by the perceiver. One interesting finding is that these brain changes occur both when subjects are purposely asked to categorize the people into social groups (e.g., to judge whether the person is Black or White), and also when they are asked to do something that draws attention away from group classifications (e.g., making a personality judgment about the person) (Ito & Urland, 2005). This tells us that we do not have to intend to make group classifications in order for them to happen. It is also very interesting to consider how quickly the changes in brain responses occur. Brain activity is altered by viewing members of different groups within 200 milliseconds of seeing a person’s face. That is just two-tenths of a second. Such a fast response lends further support to the idea that social categorization occurs automatically and may not depend on conscious intention.
Overall, this research suggests that we engage in social categorization very frequently. In fact, it appears to happen automatically (i.e., without us consciously intending for it to happen) in most situations for dimensions like gender, age, and race. Since classifying someone into a group is the first step to activating a group stereotype, this research provides important information about how easily stereotypes can be activated. And because it is hard for people to accurately report on things that happen so quickly, this issue has been difficult to study using more traditional self-report measures. Using EEGs has, therefore, been helpful in providing interesting new insights into social behavior.
Do We Use Our Own Behavior to Help Us Understand Others?
Classifying someone into a social group then activating the associated stereotype is one way to make inferences about others. However, it is not the only method. Another strategy is to imagine what our own thoughts, feelings, and behaviors would be in a similar situation. Then we can use our simulated reaction as a best guess about how someone else will respond (Goldman, 2005). After all, we are experts in our own feelings, thoughts, and tendencies. It might be hard to know what other people are feeling and thinking, but we can always ask ourselves how we would feel and act if we were in their shoes.
There has been some debate about whether simulation is used to get into the minds of others (Carruthers & Smith, 1996; Gallese & Goldman, 1998). Social neuroscience research has addressed this question by looking at the brain areas used when people think about themselves and others. If the same brain areas are active for the two types of judgments, it lends support to the idea that the self may be used to make inferences about others via simulation.
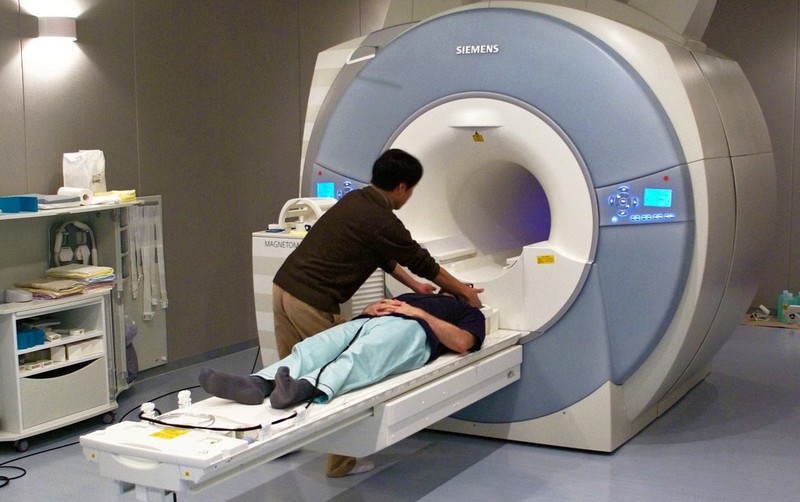
We know that an area in the prefrontal cortex called the medial prefrontal cortex (mPFC) – located in the middle of the frontal lobe – is active when people think about themselves (Kelley, Macrae, Wyland, Caglar, Inati, & Heatherton, 2002). This conclusion comes from studies using functional magnetic resonance imaging, or fMRI. While EEG measures the brain’s electrical activity, fMRI measures changes in the oxygenation of blood flowing in the brain. When neurons become more active, blood flow to the area increases to bring more oxygen and glucose to the active cells. fMRI allows us to image these changes in oxygenation by placing people in an fMRI machine or scanner (Figure 3), which consists of large magnets that create strong magnetic fields. The magnets affect the alignment of the oxygen molecules within the blood (i.e., how they are tilted). As the oxygen molecules move in and out of alignment with the magnetic fields, their nuclei produce energy that can be detected with special sensors placed close to the head. Recording fMRI involves having the subject lay on a small bed that is then rolled into the scanner. While fMRI does require subjects to lie still within the small scanner and the large magnets involved are noisy, the scanning itself is safe and painless. Like EEG, the subject can then be asked to think about different topics or engage in different tasks as brain activity is measured. If we know what a person is thinking or doing when fMRI detects a blood flow increase to a particular brain area, we can infer that part of the brain is involved with the thought or action. fMRI is particularly useful for identifying which particular brain areas are active at a given point in time.
The conclusion that the mPFC is associated with the self comes from studies measuring fMRI while subjects think about themselves (e.g., saying whether traits are descriptive of themselves). Using this knowledge, other researchers have looked at whether the same brain area is active when people make inferences about others. Mitchell, Neil Macrae, and Banaji (2005) showed subjects pictures of strangers and had them judge either how pleased the person was to have his or her picture taken or how symmetrical the face appeared. Judging whether someone is pleased about being photographed requires making an inference about someone’s internal feelings – we call this mentalizing. By contrast, facial symmetry judgments are based solely on physical appearances and do not involve mentalizing. A comparison of brain activity during the two types of judgments shows more activity in the mPFC when making the mental versus physical judgments, suggesting this brain area is involved when inferring the internal beliefs of others.
There are two other notable aspects of this study. First, mentalizing about others also increased activity in a variety of regions important for many aspects of social processing, including a region important in representing biological motion (superior temporal sulcus or STS), an area critical for emotional processing (amygdala), and a region also involved in thinking about the beliefs of others (temporal parietal junction, TPJ) (Gobbini & Haxby, 2007; Schultz, Imamizu, Kawato, & Frith, 2004) (Figure 4). This finding shows that a distributed and interacting set of brain areas is likely to be involved in social processing. Second, activity in the most ventral part of the mPFC (the part closer to the belly rather than toward the top of the head), which has been most consistently associated with thinking about the self, was particularly active when subjects mentalized about people they rated as similar to themselves. Simulation is thought to be most likely for similar others, so this finding lends support to the conclusion that we use simulation to mentalize about others. After all, if you encounter someone who has the same musical taste as you, you will probably assume you have other things in common with him. By contrast, if you learn that someone loves music that you hate, you might expect him to differ from you in other ways (Srivastava, Guglielmo, & Beer, 2010). Using a simulation of our own feelings and thoughts will be most accurate if we have reason to think the person’s internal experiences are like our own. Thus, we may be most likely to use simulation to make inferences about others if we think they are similar to us.
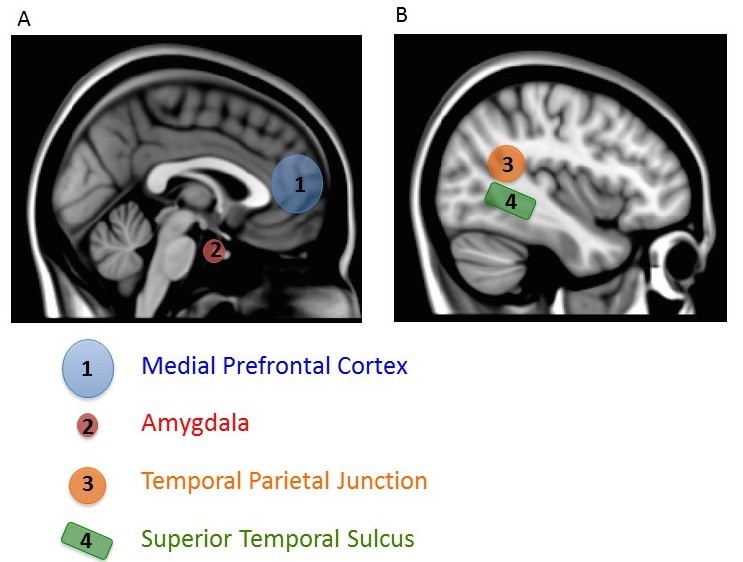
This research is a good example of how social neuroscience is revealing the functional neuroanatomy of social behavior. That is, it tells us which brain areas are involved with social behavior. The mPFC (as well as other areas such as the STS, amygdala, and TPJ) is involved in making judgments about the self and others. This research also provides new information about how inferences are made about others. Whereas some have doubted the widespread use of simulation as a means for making inferences about others, the activation of the mPFC when mentalizing about others, and the sensitivity of this activation to similarity between self and other, provides evidence that simulation occurs.
What Is the Cost of Social Stress?
Stress is an unfortunately frequent experience for many of us. Stress – which can be broadly defined as a threat or challenge to our well-being – can result from everyday events like a course exam or more extreme events such as experiencing a natural disaster. When faced with a stressor, sympathetic nervous system activity increases in order to prepare our body to respond to the challenge. This produces what Selye (1950) called a fight or flight response. The release of hormones, which act as messengers from one part of an organism (e.g., a cell or gland) to another part of the organism, is part of the stress response.
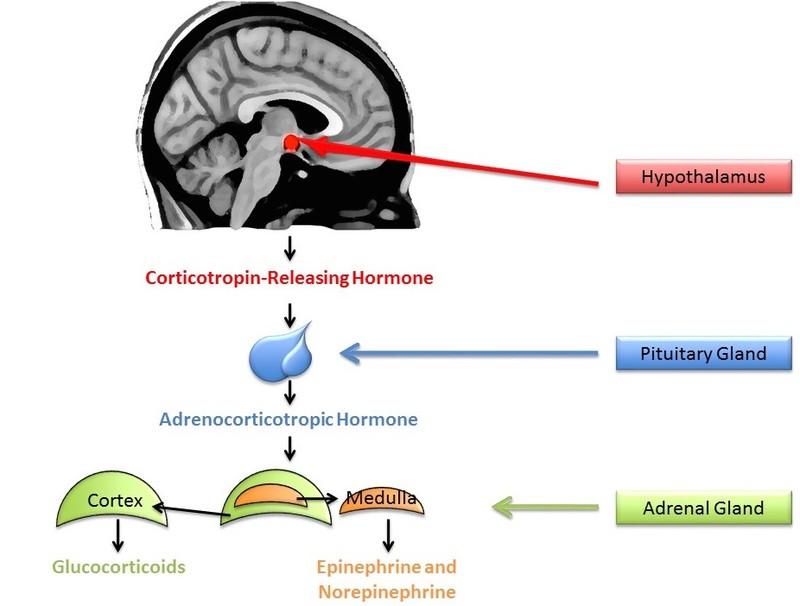
A small amount of stress can actually help us stay alert and active. In comparison, sustained stressors, or chronic stress, detrimentally affect our health and impair performance (Al’Absi, Hugdahl, & Lovallo, 2002; Black, 2002; Lazarus, 1974). This happens in part through the chronic secretion of stress-related hormones (e.g., Davidson, Pizzagalli, Nitschke, & Putnam, 2002; Dickerson, Gable, Irwin, Aziz, & Kemeny, 2009). In particular, stress activates the hypothalamic-pituitary-adrenal (HPA) axis to release cortisol (see Figure 5 for a discussion). Chronic stress, by way of increases in cortisol, impairs attention, memory, and self-control (Arnsten, 2009). Cortisol levels can be measured non-invasively in bodily fluids, including blood and saliva. Researchers often collect a cortisol sample before and after a potentially stressful task. In one common collection method, subjects place polymer swabs under their tongue for 1 to 2 minutes to soak up saliva. The saliva samples are then stored and analyzed later to determine the level of cortisol present at each time point.
Whereas early stress researchers studied the effects of physical stressors like loud noises, social neuroscientists have been instrumental in studying how our interactions with other people can cause stress. This question has been addressed through neuroendocrinology, or the study of how the brain and hormones act in concert to coordinate the physiology of the body. One contribution of this work has been in understanding the conditions under which other people can cause stress. In one study, Dickerson, Mycek, and Zaldivar (2008) asked undergraduates to deliver a speech either alone or to two other people. When the students gave the speech in front of others, there was a marked increase in cortisol compared with when they were asked to give a speech alone. This suggests that like chronic physical stress, everyday social stressors, like having your performance judged by others, induces a stress response. Interestingly, simply giving a speech in the same room with someone who is doing something else did not induce a stress response. This suggests that the mere presence of others is not stressful, but rather it is the potential for them to judge us that induces stress.
Worrying about what other people think of us is not the only source of social stress in our lives. Other research has shown that interacting with people who belong to different social groups than us – what social psychologists call outgroup members – can increase physiological stress responses. For example, cardiovascular responses associated with stress like contractility of the heart ventricles and the amount of blood pumped by the heart (what is called cardiac output) are increased when interacting with outgroup as compared with ingroup members (i.e., people who belong to the same social group we do) (Mendes, Blascovich, Likel, & Hunter, 2002). This stress may derive from the expectation that interactions with dissimilar others will be uncomfortable (Stephan & Stephan, 1985) or concern about being judged as unfriendly and prejudiced if the interaction goes poorly (Plant & Devine, 2003).
The research just reviewed shows that events in our social lives can be stressful, but are social interactions always bad for us? No. In fact, while others can be the source of much stress, they are also a major buffer against stress. Research on social support shows that relying on a network of individuals in tough times gives us tools for dealing with stress and can ward off loneliness (Cacioppo & Patrick, 2008). For instance, people who report greater social support show a smaller increase in cortisol when performing a speech in front of two evaluators (Eisenberger, Taylor, Gable, Hilmert, & Lieberman, 2007).
What determines whether others will increase or decrease stress? What matters is the context of the social interaction. When it has potential to reflect badly on the self, social interaction can be stressful, but when it provides support and comfort, social interaction can protect us from the negative effects of stress. Using neuroendocrinology by measuring hormonal changes in the body has helped researchers better understand how social factors impact our body and ultimately our health.
Conclusions
Human beings are intensely social creatures – our lives are intertwined with other people and our health and well-being depend on others. Social neuroscience helps us to understand the critical function of how we make sense of and interact with other people. This module provides an introduction to what social neuroscience is and what we have already learned from it, but there is much still to understand. As we move forward, one exciting future direction will be to better understand how different parts of the brain and body interact to produce the numerous and complex patterns of social behavior that humans display. We hinted at some of this complexity when we reviewed research showing that while the mPFC is involved in mentalizing, other areas such as the STS, amygdala, and TPJ are as well. There are likely additional brain areas involved as well, interacting in ways we do not yet fully understand. These brain areas in turn control other aspects of the body to coordinate our responses during social interactions. Social neuroscience will continue to investigate these questions, revealing new information about how social processes occur, while also increasing our understanding of basic neural and physiological processes.
Outside Resources
- Society for Social Neuroscience
- http://www.s4sn.org
- Video: See a demonstration of fMRI data being collected.
- Video: See an example of EEG data being collected.
- Video: View two tasks frequently used in the lab to create stress – giving a speech in front of strangers, and doing math computations out loud in front of others. Notice how some subjects show obvious signs of stress, but in some situations, cortisol changes suggest that even people who appear calm are experiencing a physiological response associated with stress.
- Video: Watch a video used by Fritz Heider and Marianne Simmel in a landmark study on social perception published in 1944. Their goal was to investigate how we perceive other people, and they studied it by seeing how readily we apply people-like interpretations to non-social stimuli.
Discussion Questions
- Categorizing someone as a member of a social group can activate group stereotypes. EEG research suggests that social categorization occurs quickly and often automatically. What does this tell us about the likelihood of stereotyping occurring? How can we use this information to develop ways to stop stereotyping from happening?
- Watch this video, similar to what was used by Fritz Heider and Marianne Simmel in a landmark study on social perception published in 1944, and imagine telling a friend what happened in the video. http://intentionperception.org/wp-content/uploads/2013/02/Heider_Flash.swf. After watching the video, think about the following: Did you describe the motion of the objects solely in geometric terms (e.g., a large triangle moved from the left to the right), or did you describe the movements as actions of animate beings, maybe even of people (e.g., the circle goes into the house and shuts the door)? In the original research, 33 of 34 subjects described the action of the shapes using human terms. What does this tell us about our tendency to mentalize?
- Consider the types of things you find stressful. How many of them are social in nature (e.g., are related to your interactions with other people)? Why do you think our social relations have such potential for stress? In what ways can social relations be beneficial and serve as a buffer for stress?
Vocabulary
- Amygdala
- A region located deep within the brain in the medial area (toward the center) of the temporal lobes (parallel to the ears). If you could draw a line through your eye sloping toward the back of your head and another line between your two ears, the amygdala would be located at the intersection of these lines. The amygdala is involved in detecting relevant stimuli in our environment and has been implicated in emotional responses.
- Automatic process
- When a thought, feeling, or behavior occurs with little or no mental effort. Typically, automatic processes are described as involuntary or spontaneous, often resulting from a great deal of practice or repetition.
- Cortisol
- A hormone made by the adrenal glands, within the cortex. Cortisol helps the body maintain blood pressure and immune function. Cortisol increases when the body is under stress.
- Electroencephalogram
- A measure of electrical activity generated by the brain’s neurons.
- Fight or flight response
- The physiological response that occurs in response to a perceived threat, preparing the body for actions needed to deal with the threat.
- Functional magnetic resonance imaging
- A measure of changes in the oxygenation of blood flow as areas in the brain become active.
- Functional neuroanatomy
- Classifying how regions within the nervous system relate to psychology and behavior.
- Hormones
- Chemicals released by cells in the brain or body that affect cells in other parts of the brain or body.
- Hypothalamic-pituitary-adrenal (HPA) axis
- A system that involves the hypothalamus (within the brain), the pituitary gland (within the brain), and the adrenal glands (at the top of the kidneys). This system helps maintain homeostasis (keeping the body’s systems within normal ranges) by regulating digestion, immune function, mood, temperature, and energy use. Through this, the HPA regulates the body’s response to stress and injury.
- Ingroup
- A social group to which an individual identifies or belongs.
- Lesions
- Damage or tissue abnormality due, for example, to an injury, surgery, or a vascular problem.
- Medial prefrontal cortex
- An area of the brain located in the middle of the frontal lobes (at the front of the head), active when people mentalize about the self and others.
- Mentalizing
- The act of representing the mental states of oneself and others. Mentalizing allows humans to interpret the intentions, beliefs, and emotional states of others.
- Neuroendocrinology
- The study of how the brain and hormones act in concert to coordinate the physiology of the body.
- Outgroup
- A social group to which an individual does not identify or belong.
- Simulation
- Imaginary or real imitation of other people’s behavior or feelings.
- The act of mentally classifying someone into a social group (e.g., as female, elderly, a librarian).
- A subjective feeling of psychological or physical comfort provided by family, friends, and others.
- Stereotypes
- The beliefs or attributes we associate with a specific social group. Stereotyping refers to the act of assuming that because someone is a member of a particular group, he or she possesses the group’s attributes. For example, stereotyping occurs when we assume someone is unemotional just because he is man, or particularly athletic just because she is African American.
- Stress
- A threat or challenge to our well-being. Stress can have both a psychological component, which consists of our subjective thoughts and feelings about being threatened or challenged, as well as a physiological component, which consists of our body’s response to the threat or challenge (see “fight or flight response”).
- Superior temporal sulcus
- The sulcus (a fissure in the surface of the brain) that separates the superior temporal gyrus from the middle temporal gyrus. Located in the temporal lobes (parallel to the ears), it is involved in perception of biological motion or the movement of animate objects.
- Sympathetic nervous system
- A branch of the autonomic nervous system that controls many of the body’s internal organs. Activity of the SNS generally mobilizes the body’s fight or flight response.
- Temporal parietal junction
- The area where the temporal lobes (parallel to the ears) and parieta lobes (at the top of the head toward the back) meet. This area is important in mentalizing and distinguishing between the self and others.
References
- Al’Absi, M., Hugdahl, K., & Lovallo, W. (2002). Adrenocortical stress responses and altered working memory performance. Psychophysiology, 39(1), 95–99.
- Arnsten, A. F. T. (2009). Stress signaling pathways that impair prefrontal cortex structure and function. Nature Neuroscience Reviews, 10(6), 410–422.
- Black, P. (2002). Stress and the inflammatory response: A review of neurogenic inflammation. *Brain, Behavior, & Immunity, 16*, 622–653.
- Cacioppo, J. T., & Berntson, G. G. (1992). Social psychological contributions to the decade of the brain: Doctrine of multilevel analysis. American Psychologist, 47, 1019–1028.
- Cacioppo, J. T., & Patrick, B. (2008). Loneliness: Human nature and the need for social connection. New York, NY: W. W. Norton & Company.
- Carruthers, P. and Smith, P. (1996). Theories of Theories of Mind. New York, NY: Cambridge University Press.
- Davidson, R. J., Pizzagalli, D., Nitschke, J. B., & Putnam, K. (2002). Depression: Perspectives from affective neuroscience. Annual Review of Psychology, 53, 545–574.
- Dickerson, S. S., Gable, S. L., Irwin, M. R., Aziz, N., & Kemeny, M. E. (2009). Social-evaluative threat and proinflammatory cytokine regulation an experimental laboratory investigation. Psychological Science, 20, 1237–1244.
- Dickerson, S. S., Mycek, P. J., & Zaldivar, F. (2008). Negative social evaluation, but not mere social presence, elicits cortisol responses to a laboratory stressor task. Health Psychology, 27(1), 116–121.
- Eisenberger, N. I., Taylor, S. E., Gable, S. L., Hilmert, C. J., & Lieberman, M. D. (2007). Neural pathways link social support to attenuated neuroendocrine stress responses. Neuroimage, 35(4), 1601–1612.
- Gallese, V., & Goldman, A. (1998). Mirror neurons and the simulation theory of mind-reading. Trends in Cognitive Sciences, 2, 493–501.
- Gobbini, M. I., & Haxby, J. V. (2007). Neural systems for recognition of familiar faces. Neuropsychologia, 45(1), 32–41.
- Goldman, A. I. (2005). Imitation, mind reading, and simulation. In S. Hurley & N. Chater (Eds.), Perspectives on imitation: From neuroscience to social science (Vol. 2: Imitation, human development, and culture, pp. 79–93). Cambridge, MA: MIT Press.
- Ito, T. A., & Urland, G. R. (2003). Race and gender on the brain: Electrocortical measures of attention to race and gender of multiply categorizable individuals. Journal of Personality and Social Psychology, 85, 616–626.
- Ito, T.A., & Urland, G.R. (2005). The influence of processing objectives on the perception of faces: An ERP study of race and gender perception. Cognitive, Affective, and Behavioral Neuroscience, 5, 21–36.
- Kelley, W. M., Macrae, C. N., Wyland, C. L., Caglar, S., Inati, S., & Heatherton, T. F. (2002). Finding the self? An event-related fMRI study. Journal of Cognitive Neuroscience, 14, 785–794.
- Lazarus, R. S., (1974). Psychological stress and coping in adaptation and illness. *International Journal of Psychiatry in Medicine, 5*, 321–333.
- Mendes, W. B., Blascovich, J., Lickel, B., & Hunter, S. (2002). Challenge and threat during social interactions with White and Black men. Personality and Social Psychology Bulletin, 28, 939–952.
- Mitchell, J. P., Neil Macrae, C., & Banaji, M. R. (2005). Forming impressions of people versus inanimate objects: social-cognitive processing in the medial prefrontal cortex. Neuroimage, 26(1), 251–257.
- Mouchetant-Rostaing, Y., & Giard, M. H. (2003). Electrophysiological correlates of age and gender perception on human faces. Journal of Cognitive Neuroscience, 15, 900–910.
- Phelps, E. A., & LeDoux, J. E. (2005). Contributions of the amygdala to emotion processing: From animal models to human behavior. Neuron, 48, 175.
- Plant, E. A., & Devine, P. G. (2003). The antecedents and implications of interracial anxiety. *Personality and Social Psychology Bulletin, 29*, 790–801.
- Schultz, J., Imamizu, H., Kawato, M., & Frith, C. D. (2004). Activation of the human superior temporal gyrus during observation of goal attribution by intentional objects. Journal of Cognitive Neuroscience, 16, 1695–1705.
- Selye, H. (1950). The physiology and pathology of exposure to stress. Montreal: Acta Inc.
- Srivastava, S., Guglielmo, S., & Beer, J. S. (2010). Perceiving others’ personalities: Examining the dimensionality, assumed similarity to the self, and stability of perceiver effects. Journal of Personality and Social Psychology, 98, 520.
- Stephan, W. G., & Stephan, C. W. (1985). Intergroup anxiety. Journal of Social Issues, 41(3), 157–175.
- Whalen, P. J., & Phelps, E. A. (2009). The human amygdala. New York, NY: The Guilford Press.
Authors
 Tiffany A. ItoTiffany A. Ito is a Professor of Psychology and Neuroscience at the University of Colorado Boulder. Her research integrates neuroscience methods and theories to better understand social processes, with a particular focus on aspects of stereotyping and prejudice.
Tiffany A. ItoTiffany A. Ito is a Professor of Psychology and Neuroscience at the University of Colorado Boulder. Her research integrates neuroscience methods and theories to better understand social processes, with a particular focus on aspects of stereotyping and prejudice. Jennifer T. KubotaJennifer Kubota is an assistant professor at University of Delaware. She received her Ph.D. in social neuroscience from The University of Colorado Boulder. Her work focuses on the psychological and neural substrates of impression formation and their relation to decision-making.
Jennifer T. KubotaJennifer Kubota is an assistant professor at University of Delaware. She received her Ph.D. in social neuroscience from The University of Colorado Boulder. Her work focuses on the psychological and neural substrates of impression formation and their relation to decision-making.
Creative Commons License



 Social Neuroscience by Tiffany A. Ito and Jennifer T. Kubota is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. Permissions beyond the scope of this license may be available in our Licensing Agreement.
Social Neuroscience by Tiffany A. Ito and Jennifer T. Kubota is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. Permissions beyond the scope of this license may be available in our Licensing Agreement.